Clinical Study
SUPERIA – Post Marketing Surveillance Study
Overview
The study was a prospective, multicenter, post marketing surveillance study to evaluate the safety and effectiveness of the Superia-Sirolimus Eluting Coronary Stent System (SSECSS) implanted during routine clinical practice in India i.e.
- 200 patients at 16 Sites all across India
- The study was led by Dr. Praveen Chandra, Chairman, Interventional Cardiology at Medanta, the Medicity Hospital
- Clinical follow-up at 30 days, 180 days, 1 year and 2 year
- Angiographic follow-up (sub-set of 50 patients) at 180 days
- Available stent sizes: 2.5, 2.75, 3.0 and 3.5 mm diameters in lengths of 12, 13, 16, 19, 20, 24, 28, 29 and 32 mm
- Inclusion Criteria:-
- Up to two de novo lesions, each located in a separate native epicardial vessel
- Maximum lesion length up to 28 mm
- Stent’s diameter 2.50 mm to 3.50 mm
- Exclusion Criteria:-
- LVEF < 30%, Heavy calcification, extreme angulation (>90%), target vessel containing thrombus with re-stenosis of previous intervention
- CK/CKMB/Troponin more than 2 times URL at the time of index procedure
- DES’s treatment within 90 days prior to index procedure
Primary Objective
MACE defined as Composite endpoint of Cardiac death and Myocardial infarction (MI) and Target Lesion Revascularization (TLR)- Time Frame – 30 days.
Co-primary objective
In stent and In-segment Late Loss at Six Months (in a per selected group of 50 patients)
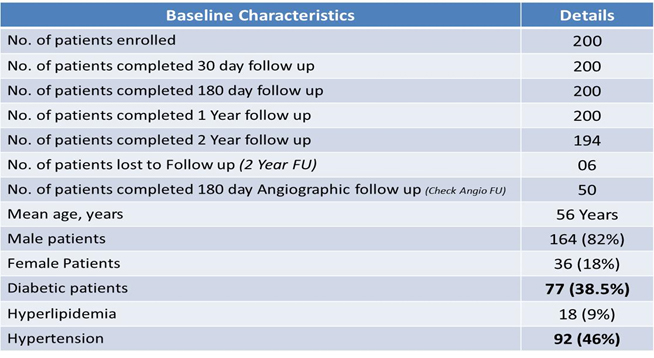
Baseline Demographics
Result
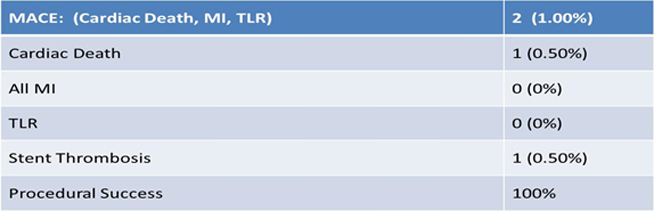
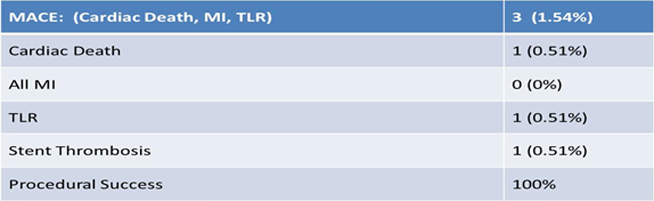
A total of 200 patients enrolled across 16 centers in India. First patients was enrolled in May 2012. Mean age of patients were 56 yr, 82% were male, 38.5% were diabetic, 46% were hypertensive and 27% were having previous MI. Procedural success was achieved in all patients. At 2 year of follow up TLR was required only in 1 patient, stent thrombosis in 1 patient and there was only 1 cardiac death and MACE was seen in 3 (1.54%).
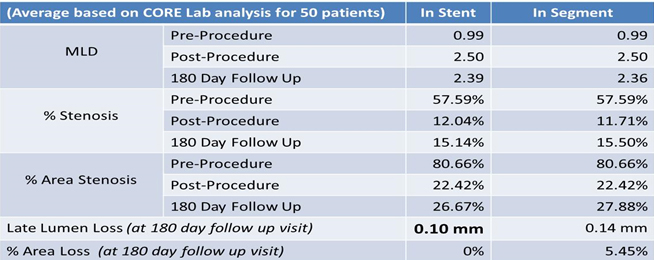
Angiographic follow was done in 50 patients. Late lumen loss studied at 6 months of follow up. In stent lumen loss was 0.10 mm and in segment lumen loss was 0.14 mm at 6 months of follow up.
End Point Analysis (30 Days, N=200)
End Point Analysis (2 Year, N = 194)
Core Lab Analysis (Pre, Post procedure and 6 month Angiographic follow-up, N=50)